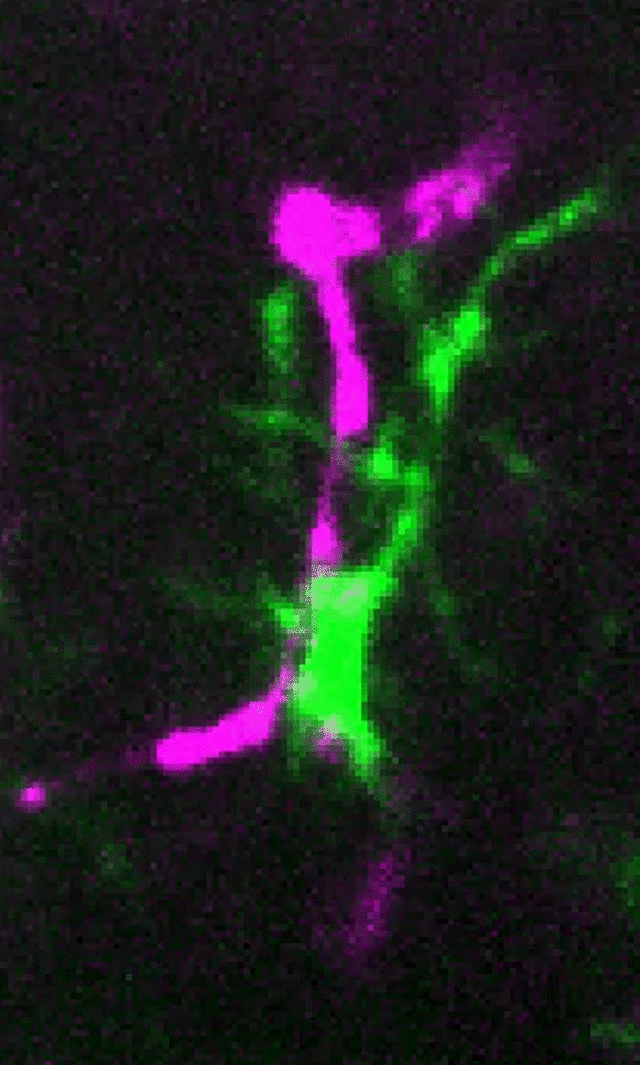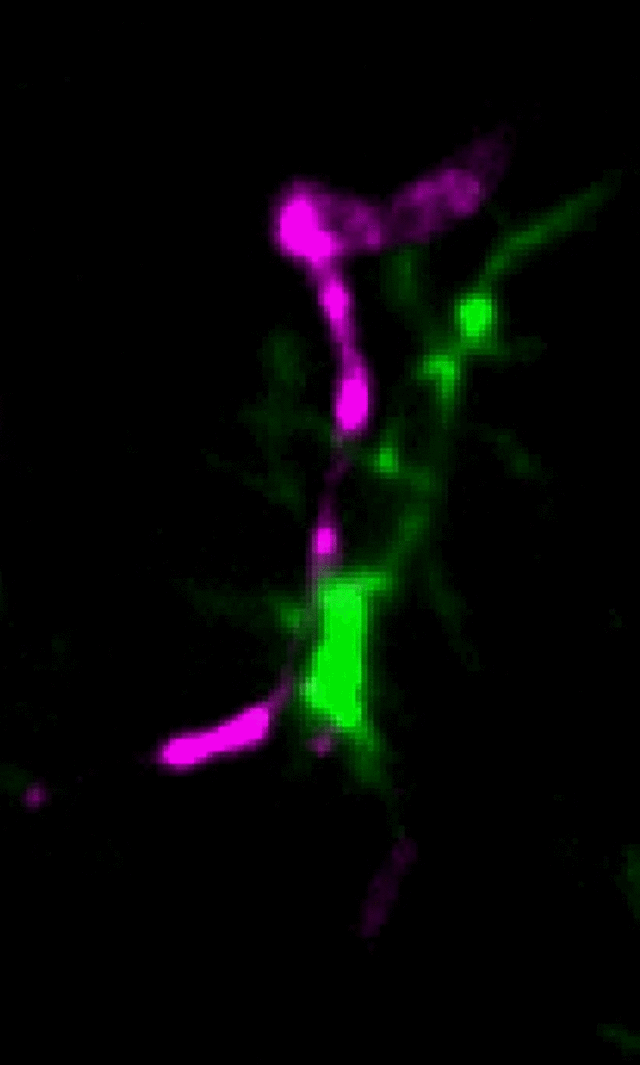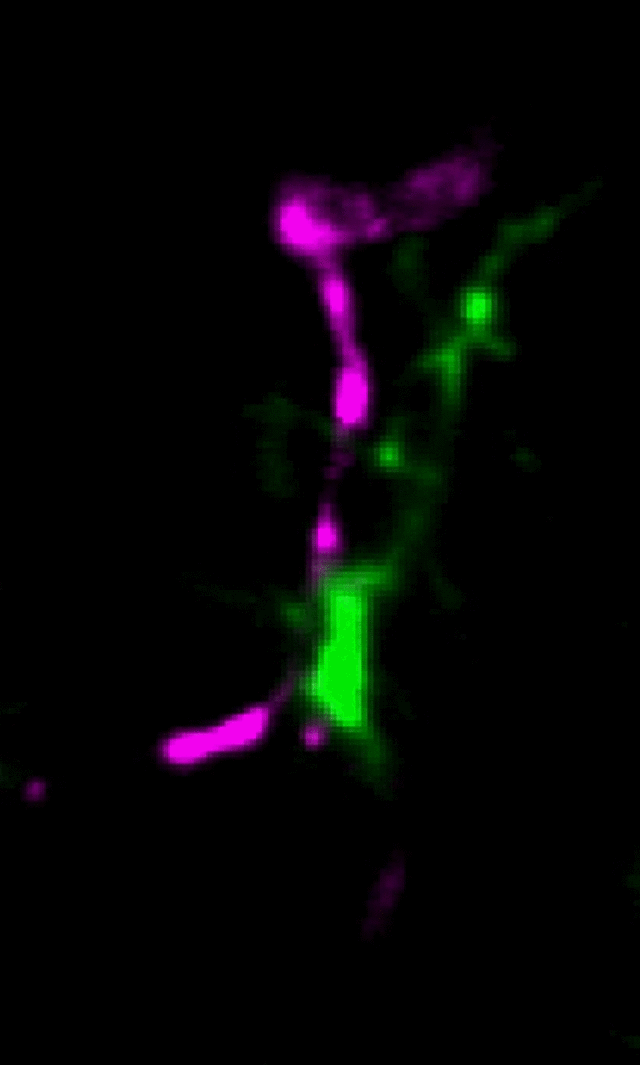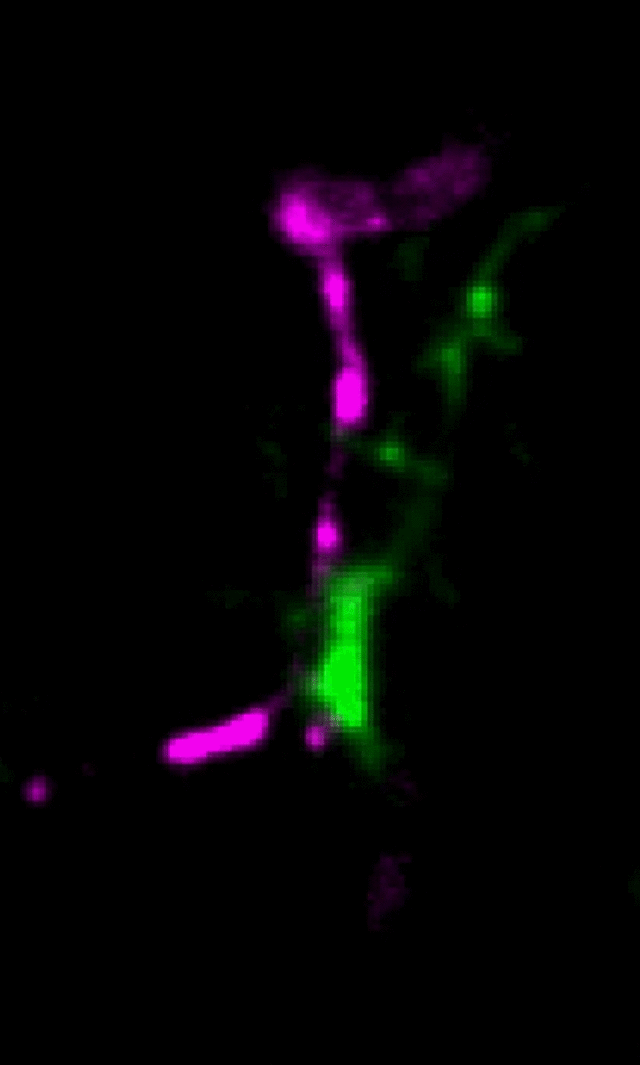
Microglia (green) and synaptic compartments (magenta) in the juvenile mouse cortex.
The primary focus of our research is to understand the role of microglia in neural circuit development and plasticity in the healthy and diseased nervous system. We are particularly interested in:
1) How do microglia respond to changes in neural activity and, in turn, modulate synaptic connectivity?
2) Is microglial dysfunction at synapses an underlying cause of neurological disease?
3) How do microglia modulate other glial and vascular cell types within the nervous system to affect brain development?
To address these questions, the lab has developed cutting-edge molecular genetic approaches combined with high resolution static and 2-photon live imaging. Major lines of research include:
Dissecting the molecular mechanisms underlying activity-dependent microglia-synapse interactions
Microglia respond to changes in neural activity and modulate synaptic connectivity but the underlying mechanisms are just beginning to be deciphered. Projects in the lab are designed to identify how microglia ‘listen’ to changes in neural activity and, in turn, modify synapse structure and function. These projects combine cutting-edge RNA sequencing approaches and novel in vitro and in vivo molecular genetic approaches to modify microglia function at synapses and determine the overall impact on neural circuit function. To visualize responses on a cellular level, we are also developing strategies to simultaneously visualize glial cells and specific synaptic circuits known to undergo experience-dependent remodeling by static, high-resolution imaging as well as live imaging in behaving mice by 2-photon microscopy.
A-B. Removal of whiskers (-whiskers) leads to decreased neural activity and corresponding loss of synapses in the barrel cortex (A) and microglial engulfment of synapses (yellow arrows, B). C. Blocking microglial engulfment in mice deficient in fractalkine receptor (CX3CR1 KO) or fractalkine ligand (CX3CL1 KO) signaling results in failure of synapses to be eliminated in the barrel cortex following whisker removal. D. We are using single-cell RNAseq approaches to dissect the cellular sources of molecules regulating microglial pruning of synapses and determine how these cell populations change with dampened neural activity following whisker removal. See also Gunner et al. Nat Neurosci 2019.
Investigating microglia-vascular interactions
Compared to neurons and other glial cells, far less is known about how microglia interact with and regulate the vasculature. We are investigating a unique population of microglia called juxtavascular microglia that are tightly associated with the CNS vasculature. Using live imaging we have shown that these juxtavascular microglia are highly motile and migrate along the vasculature during brain development and then upon adulthood they become highly stable. We are now using spatial transcriptomic approaches and further 2-photon live imaging to better understand these juxtavascular microglial cells in health and disease.
Neonate
Adult
Left top panel, a juxtavascular microglia (green) in the neonate cortex during the first postnatal week. Right top panels, videos of live imaging of juxtavascular microglia in acute brain slices over 6 hrs. Juxtavascualar microglia are highly migratory along vessels in neonates (middle top panel) and stable in adults (right top panel). Bottom panel, 2-photon live imaging in awake, behaving adult mice demonstrates that juxtavascular microglia (green, arrowheads) are highly stable over 6 weeks of imaging. See Mondo et al. J. Neurosci 2020
Dissect how microglia contribute to synaptic changes in neurological disorders
It is now appreciated that abnormal synaptic connectivity is an underlying feature of neurodevelopmental and psychiatric disorders such as autism and schizophrenia. Further, early synapse loss is an underlying feature and, perhaps causative in an array of neurodegenerative diseases (Alzheimer’s disease, ALS, Parkinson’s, Multiple Sclerosis, etc.). Projects in the lab include using human-derived microglia-like cells to investigate microglial inflammatory signaling and synapse pruning functions relevant to autism spectrum disorders. We are also assessing how microglia modulate synaptic connectivity at early stages of neurodegeneration with a focus on multiple sclerosis and Alzheimer’s disease.
Top panel, Synapse (immunostained with anti-VGluT2) loss in the human MS thalamus (left) and Iba+ putative microglia engulfment of presynaptic terminals (right, white arrows highlight engulfed material). This biology is recapitulated in a mouse model of inflammatory demeyleinating disease (mouse EAE). If we block microglial engulfment, we can prevent synapse loss (see Werneburg et al. Immunity 2020)